Varda Shine's Meet and Greet session with Mumbai media also outlines De Beers plans for 2011
Samples of CVD diamonds grown during the same run at LIMHP-CNRS. Colors range from D to F and thickness vary from 0.384 mm to 0.785 mm. After substrate removal and polishing, this corresponds to weights of 0.097 carats to 0.265 carats.
Photographs of a CVD diamonds plate from LIMHP-CNRS (0.48 mm thick after substrate removal and polishing, 0.25 carats, D-color, with crossed-polarizers showing birefrigence features in a cross shape. Those birefringence features are characteristic of CVD samples and run all the way through the sample.
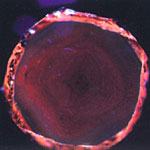
A DiamondView image of a CVD diamond plate from LIMHP-CNRS (0.50 mm thick after substrate removal and polishing, 0.12 carats, E-color.
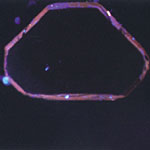
A DiamondView image of a CVD diamond plate from IMO (0.27 mm thick after substrate removal and polishing, 0.03 carats, E-color.
Entry Into the Gems Sector not far away:
The initial announcement by U.S. based Apollo Diamond that it would be commercializing synthetic gem diamonds grown by the {{Chemical Vapor Deposition}} ({{CVD}}) process rocked the diamond sector.%%
Interestingly, the knowledge of CVD, which involves carbon atoms precipitating onto a diamond seed under low pressure in a special reactor chamber, dates back to the 1950s. Important players, such as {{General Electric}} ({{GE}}), {{De Beers}} and {{Sumitomo}}, pushed forward with synthetic research using CVD techniques and more conventional {{High Pressure High Temperature}} ({{HPHT}}) processes, mainly for industrial applications.
CVD Gem-Diamonds Round the Corner:
In contrast to HPHT-synthetic diamonds, CVD gem-quality stones are not yet available commercially, but as research groups develop the know-how, gem-quality CVD diamonds will surely claim entry into the diamond sector. It was therefore felt crucial that research should characterize and identify diamonds created by the CVD method.%%
In order to gain access to CVD material, with the aim of adding to our body of knowledge, {{HRD Research}} created several close collaborations with CVD-producing groups. Among them were partnerships with a team from Paris University 13 (LIMHP-CNRS) of France, led by Prof. A Gicquel, and another with the {{Instituut voor Materiaal Onderzoek}} ({{IMO}}) from the University of Hasselt in Belgium. Although they both grow CVD diamonds only for scientific purposes, a study of their production methods provided good insight into the progress of CVD growth.
Recently HRD Research examined eight CVD samples provided by the research team from LIMHP-CNRS and seven samples provided by the IMO group. They were analyzed using UV topography (Diamond View TM), D-Scope under polarized light, infrared spectroscopy, UVVIS spectroscopy, Photoluminescence spectroscopy, Gran (color determination) and Diamond Sure TM.%%
The single crystal CVD diamond films, grown at IMO on 2.5 x 2.5 mm HPHT substrates were synthesized using a commercial microwave plasma enhanced CVD system from ASTeX, U.S or SEKI AX6000 from Japan. The substrate holder, developed at IMO, allows the application to be conducted under relatively low powers with high gas pressure of typically 200 torr (a unit of pressure equal to 0.001316 atmospheres).
%%
This surprisingly leads to the growth of diamond films, with a thickness of between 0.5 mm and 1.0 mm, with almost atomically flat surfaces. The plasma is used in the continuous mode.
%%
The CVD reactor used at LIMHP-CNRS is homemade. A microwave power density in the range 75-190 W/cm3(watts per cubic centimetre) is coupled to the plasma in either a continuous or a pulsed mode, allowing a deposition rate as high purity thick monocrystalline diamond film as thick as 1.7 mm.
One of our most striking observations is that LIMHP-CNRS managed to improve significantly the "best" color of its CVD diamonds in a period of less than a year. HRD Research compared the latest set of material that we received to the six samples produced by LIMHP-CNRS during late 2004 and early 2005, which were described in the Fall 2005 issue of Gems and Gemology (p.234-244). It shows that progress from G color to D color was achieved. It is further interesting to note that excellent colors can be achieved both in nitrogen doped runs and in high-purity runs.
%%
At IMO, optimal growth parameters are currently being investigated. Each run consists of the growth of a single sample. At LIMHP-CNRS, multiple growth experiments are conducted as well and up to three samples may be grown during the same run (Figure 1).%%
At present typical growth speeds for CVD monocrystals are in the order of 0.005 to 0.1 mm per hour.
%%
In May 2005 it was revealed by the U.S. based {{Carnegie Institution}} that a 10-carat CVD single-crystal diamond had been synthesized in just several hours. To diamantaires this may sound frightening, but in fact the large size was obtained by growing sequentially 6 faces on a substrate diamond plate. The growth rate corresponded to 0.1 mm per hour and the resulting CVD stone was brownish.%%
CVD synthetic diamonds range in color from brown to colorless with the latter the most difficult to grow. In case the growth speed is increased by adding nitrogen, a relationship exists between the growth rate and the resulting color-the higher the growth rate, the browner the sample.%%
It is obvious that maintaining good colors in thick samples is the challenge for researchers active in CVD synthesis. Representatives of Apollo recently showed HRD Research colorless CVD brilliants of 0.3 carats, in order to demonstrate their capacity.
It is important to note that the commercially available detection instruments, such as D-Screen, which is produced by HRD Equipment (Comdiam), and DiamondSureTM produced by the Diamond Trading Company (DTC), refer all colorless and near-colorless CVD diamonds for further testing.
%%
Moreover, important telltale-signs are found by viewing CVD crossed polarizers. Cross-like features like the one shown in Figure 2 are encountered in CVD. They are observed through the stone.
%%
Another feature of CVD diamond that may be helpful for detection is the obvious reddish appearance of most stones when investigated by DiamondViewTM (Figure 3,4).
%%
In addition, CVD is traceable by photoluminescence spectroscopy in gemmological laboratories.
CVD diamonds are seen by many to be a threat, but it is reassuring that identifying CVD stones has thus far posed no problem to HRD Research. Ongoing investigation is the key message, and HRD Research will continue to invest in identification techniques.
CVD is not a new technique. It fact, it was the first method employed to successfully grow synthetic diamonds in 1952, when {{William Eversole}} of the Union Carbide company decomposed carbon monoxide gas at low pressures in a sealed vessel in the presence of diamond seed crystals. A diamond film did form during this process, but at the time it was considered to be too slow to have a commercial impact. Eversole made this breakthrough just a few months before General Electric created its first diamonds using an HPHT process.%%
CVD is a method that deposits diamond from a hot gas onto a cold substrate, much like frost gathers on a cold window in the winter. In this case "cold" is a relative term, inasmuch as the substrate has a temperature of about 800 degrees Celsius, which, nevertheless, is significantly colder than the gases contained in the plasma in the CVD reactor.%%
To grow diamonds the gas needs to contain carbon atoms. But, whereas Eversole used carbon monoxide (CO), nowadays, in most CVD systems the carbon atoms are delivered by methane (CH4). They consist of one carbon atom bound to four hydrogen atoms. At high temperatures, such as those obtained in the CVD reactor, these molecules dissociate (break up) and carbon atoms are free to reconstruct on the substrate. Not only diamond is formed in this way but also graphite. To solve this problem hydrogen gas (H2) is added. These atoms etch the graphite on the substrate more than they do diamond. The result is that only diamond grows on the substrate.


Be the first to comment